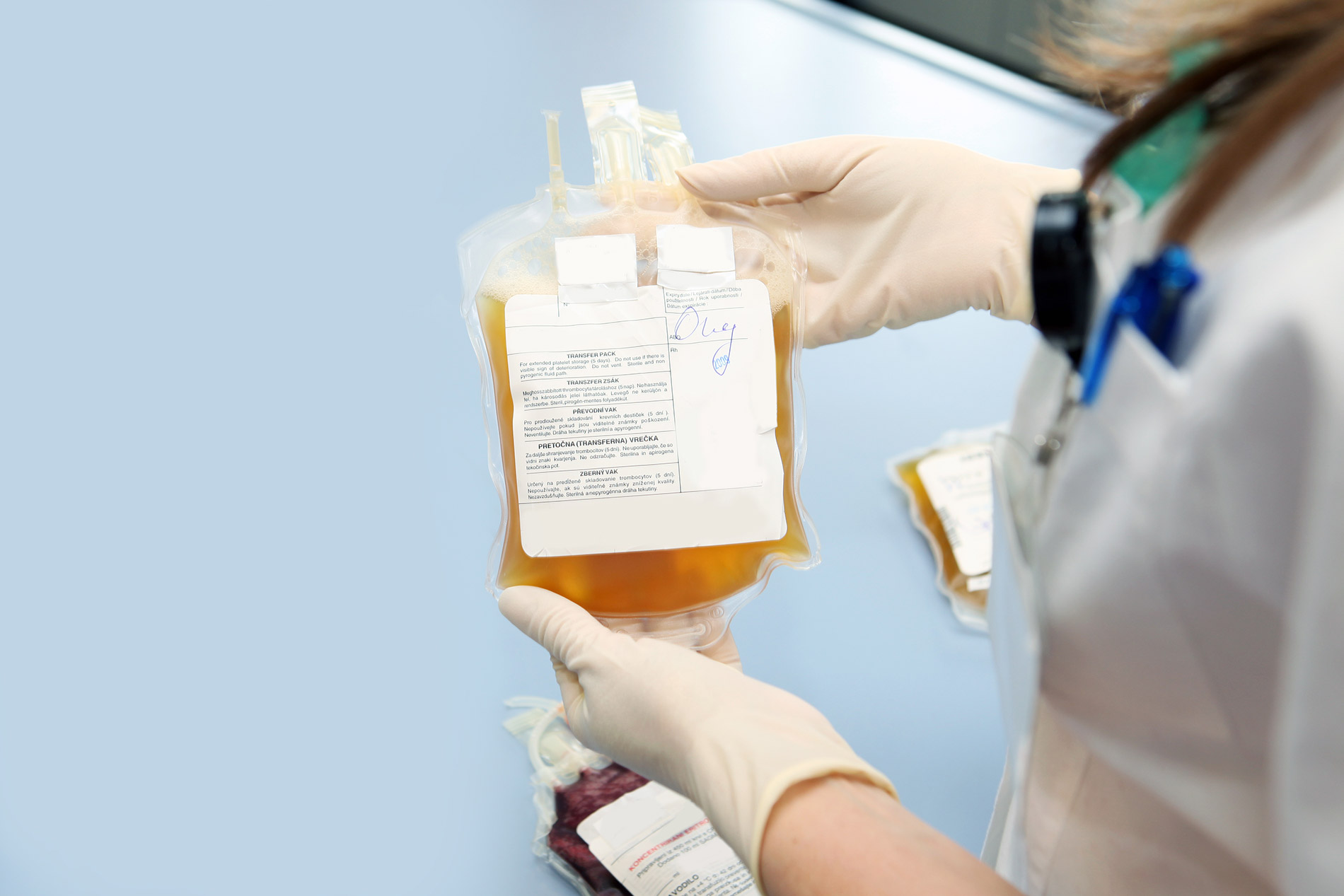
Carle Health and Community Blood Services of Illinois, are collaborating on an investigational treatment with a century-old approach to treat hospitalized COVID-19 patients.
First Community Blood Services of Illinois collects plasma from a recovered COVID-19 donor. Then, physicians use it to treat the sickest COVID-19 patients. Carle is the first hospital in the region to administer the plasma to patients.
 Researchers hope the treatment, called convalescent plasma, will boost the ability of people with severe COVID-19 symptoms to fight the virus more effectively.
Researchers hope the treatment, called convalescent plasma, will boost the ability of people with severe COVID-19 symptoms to fight the virus more effectively.
“The immediate goal is to determine if convalescent plasma can improve the chance of recovery for people with the most severe illness. A second goal is to test whether convalescent plasma can help keep people who are moderately sick from becoming sicker,” said Mark Johnson, MD, Critical Care.
The collaboration is part of a Mayo Clinic clinical trial.
Dr. Louis Katz, an infectious disease specialist and chief medical officer with Community Blood Services, expressed cautious optimism.
“This therapy is unproven. There are historic precedents that suggest modest efficacy in a number of other infectious diseases, including influenza. Plasma is used routinely in transfusion, where it has an excellent safety profile that we expect to be maintained with this product. Very preliminary data from China suggest that will be the case, but as yet there are no valid clinical outcome studies of convalescent plasma for COVID-19,” said Katz, adding, “We are hopeful.”
Karen White, MD, PhD, the principal investigator for the trial at Carle said the convalescent plasma isn’t new method and has treated outbreaks including SARS, Ebola, H1N1 flu and measles, but using it to treat COVID-19 has not yet been proven effective.
“Carle physicians are committed to providing the safest and also most up to date care for the COVID-19 patient,” she said.
Dr. White said initial data from studies using COVID-19 convalescent plasma for individuals with severe or life-threatening disease have showed potential positive results and buys researchers more time to develop a true vaccine.
 “This kind of trial and its potential for healing keeps us motivated and full of hope for the future,” said Jennifer Eardley, Carle vice president of Research. “This is a scarce resource right now and can be difficult to acquire so we’re encouraged by our ability to partner and meet this need.”
“This kind of trial and its potential for healing keeps us motivated and full of hope for the future,” said Jennifer Eardley, Carle vice president of Research. “This is a scarce resource right now and can be difficult to acquire so we’re encouraged by our ability to partner and meet this need.”
Bruce Wellman, MD, Laboratory and Pathology Services, said our blood center partners and teams moved quickly to bring this to our patients and the region.
“We can potentially help patients breathe easier without the aid of a ventilator or get them out of the hospital and on the road to recovery,” Dr. Johnson said.
Dr. Wellman said those who have already recovered from the virus are encouraged to donate. Community Blood Services of Illinois supplies Carle with blood products given by volunteer donors. Convalescent plasma donations made locally and across the Blood Center’s service region will make this additional product available for patient care.
“For those who have had COVID-19 and recovered, this is the greatest way to give back,” Dr. Wellman said.
Carle’s research team is contacting fully recovered patients at the appropriate time (at least 28 days following their full recovery) to inform them about their potential eligibility and to assist them in getting their required documentation and scheduling appointments.
Visit Community Blood Services of Illinois at bloodcenter.org for more information or reach out to patientservices@mvrbc.org or call (833) 610-1025.
First Community Blood Services of Illinois collects plasma from a recovered COVID-19 donor. Then, physicians use it to treat the sickest COVID-19 patients. Carle is the first hospital in the region to administer the plasma to patients.
 Researchers hope the treatment, called convalescent plasma, will boost the ability of people with severe COVID-19 symptoms to fight the virus more effectively.
Researchers hope the treatment, called convalescent plasma, will boost the ability of people with severe COVID-19 symptoms to fight the virus more effectively. “The immediate goal is to determine if convalescent plasma can improve the chance of recovery for people with the most severe illness. A second goal is to test whether convalescent plasma can help keep people who are moderately sick from becoming sicker,” said Mark Johnson, MD, Critical Care.
The collaboration is part of a Mayo Clinic clinical trial.
Dr. Louis Katz, an infectious disease specialist and chief medical officer with Community Blood Services, expressed cautious optimism.
“This therapy is unproven. There are historic precedents that suggest modest efficacy in a number of other infectious diseases, including influenza. Plasma is used routinely in transfusion, where it has an excellent safety profile that we expect to be maintained with this product. Very preliminary data from China suggest that will be the case, but as yet there are no valid clinical outcome studies of convalescent plasma for COVID-19,” said Katz, adding, “We are hopeful.”
Karen White, MD, PhD, the principal investigator for the trial at Carle said the convalescent plasma isn’t new method and has treated outbreaks including SARS, Ebola, H1N1 flu and measles, but using it to treat COVID-19 has not yet been proven effective.
“Carle physicians are committed to providing the safest and also most up to date care for the COVID-19 patient,” she said.
Dr. White said initial data from studies using COVID-19 convalescent plasma for individuals with severe or life-threatening disease have showed potential positive results and buys researchers more time to develop a true vaccine.
 “This kind of trial and its potential for healing keeps us motivated and full of hope for the future,” said Jennifer Eardley, Carle vice president of Research. “This is a scarce resource right now and can be difficult to acquire so we’re encouraged by our ability to partner and meet this need.”
“This kind of trial and its potential for healing keeps us motivated and full of hope for the future,” said Jennifer Eardley, Carle vice president of Research. “This is a scarce resource right now and can be difficult to acquire so we’re encouraged by our ability to partner and meet this need.”Bruce Wellman, MD, Laboratory and Pathology Services, said our blood center partners and teams moved quickly to bring this to our patients and the region.
“We can potentially help patients breathe easier without the aid of a ventilator or get them out of the hospital and on the road to recovery,” Dr. Johnson said.
Dr. Wellman said those who have already recovered from the virus are encouraged to donate. Community Blood Services of Illinois supplies Carle with blood products given by volunteer donors. Convalescent plasma donations made locally and across the Blood Center’s service region will make this additional product available for patient care.
“For those who have had COVID-19 and recovered, this is the greatest way to give back,” Dr. Wellman said.
Carle’s research team is contacting fully recovered patients at the appropriate time (at least 28 days following their full recovery) to inform them about their potential eligibility and to assist them in getting their required documentation and scheduling appointments.
Visit Community Blood Services of Illinois at bloodcenter.org for more information or reach out to patientservices@mvrbc.org or call (833) 610-1025.
Categories: Culture of Quality
Tags: coronavirus, COVID-19, healthy, plasma, research, testing