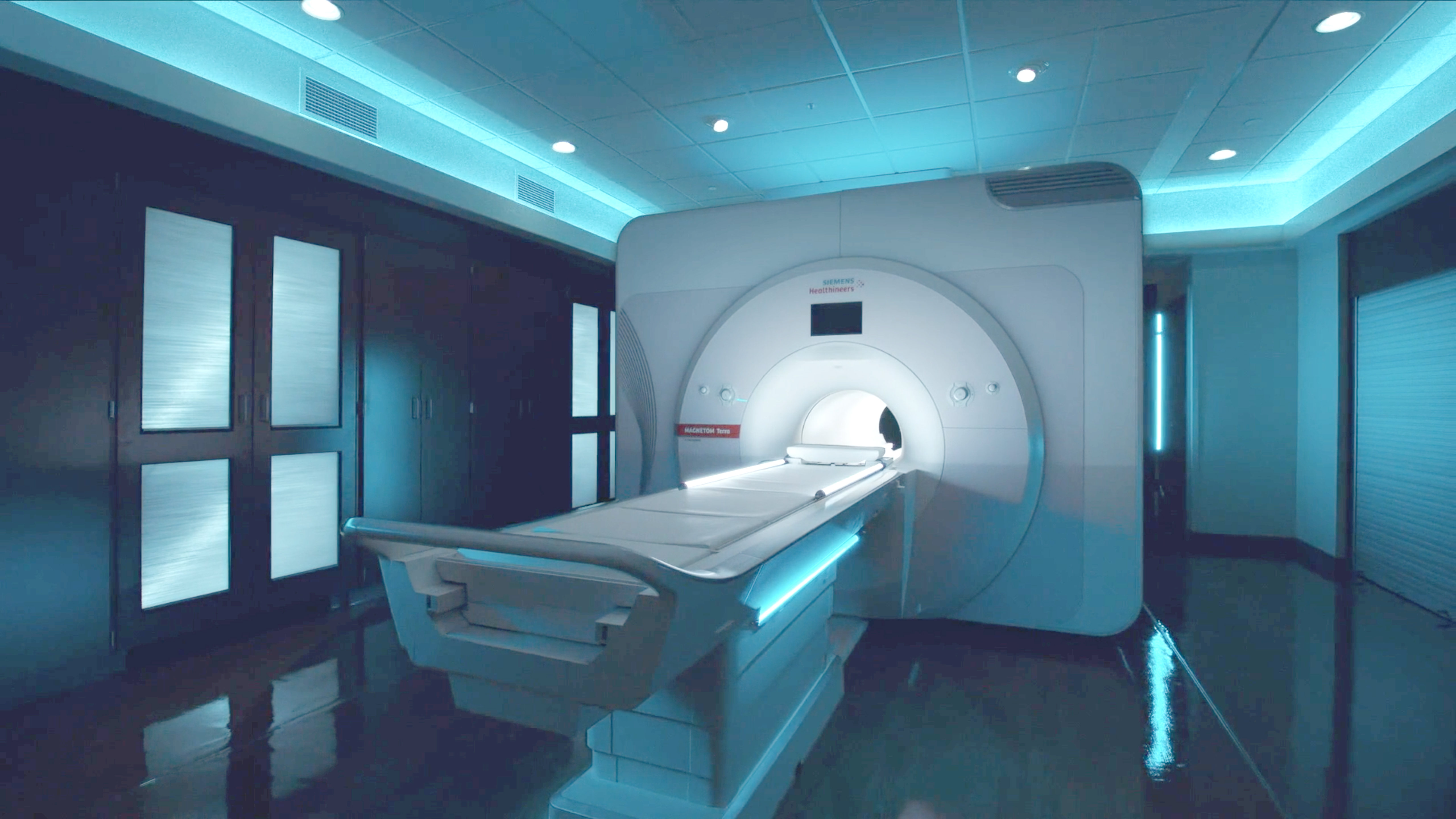
A student-faculty research collaboration at Carle Illinois College of Medicine offers clinicians new insight into which neurosurgical implant devices have proven compatible with 7Tesla (7T) magnetic resonance imaging (MRI) scanning. Their findings could influence clinicians to consider using the advanced, high resolution MRI technology to benefit patients who already have certain implants.
7T is considered state-of-the-art MRI technology. In the few locations where 7T MRI is available, its ultra-high-resolution images can be helpful in diagnosing brain tumors prior to surgery, identifying small lesions within the brain, and in diagnosing and planning surgery across specialties. But many patients with existing neurosurgical implants (such as aneurysm clips, shunts for treating fluid build-up in the brain, and hardware for spinal surgery) are denied the benefits of 7T scanning out of compatibility and safety concerns.
A research team made up of Carle Illinois medical students, faculty, researchers from the Beckman Institute for Advanced Science and Technology, and clinicians at Carle Health is the first to delve into which of these devices has been tested with 7T MRI scanning. “We found that many implants have been tested at lower MRI fields (1.5T, 3T), but few have been adequately tested at 7T MRI,” said Annabelle Shaffer, a second-year medical student at Carle Illinois and study co-author. The testing results are particularly limiting in the clinical setting because patients with known neurosurgical issues are among the most likely to need MR imaging.
Advanced Science and Technology, and clinicians at Carle Health is the first to delve into which of these devices has been tested with 7T MRI scanning. “We found that many implants have been tested at lower MRI fields (1.5T, 3T), but few have been adequately tested at 7T MRI,” said Annabelle Shaffer, a second-year medical student at Carle Illinois and study co-author. The testing results are particularly limiting in the clinical setting because patients with known neurosurgical issues are among the most likely to need MR imaging.
While the lack of widespread compatibility testing is discouraging, the news is better for some patients with certain neurosurgical implants. “We found that cranial fixation devices, deep brain stimulation devices, spinal rods, and pedicle screws are likely 7T MRI-compatible based on outcomes reported,” Shaffer said.
In 2021, Carle Foundation Hospital in Urbana became one of only a few hospitals in the country and the first in Illinois, to acquire the 7T MRI for both clinical and research use through a partnership with the University of Illinois Urbana-Champaign.
Shaffer said the team’s research is timely and may encourage implant device manufacturers to ensure that their devices are compatible with 7T and to test their products, using industry standards. “We hope more patients with biomedical implants (regardless of location) will be able to be safely imaged with this exciting technology,” Shaffer said.
The research team’s article “Neurosurgical Implant Safety in 7 T MRI: A Scoping Review,” was recently published in the Journal of Magnetic Resonance Imaging. Team members included: CI MED student Anant Naik; Carle Illinois College of Medicine Dean Dr. Mark Cohen; Dr. Paul M Arnold, a neurosurgeon at Carle Health and professor at Carle Illinois College of Medicine; Professor Brad Sutton of Carle Illinois College of Medicine and the Beckman Institute; Dr. David Weisbaum of Carle Health’s Department of Neurosurgery; and Aaron Anderson, Tracey Wszalek, Andrew Webb, and Bruce Damon of the Carle Illinois Advanced Imaging Center and the Beckman Institute.
7T is considered state-of-the-art MRI technology. In the few locations where 7T MRI is available, its ultra-high-resolution images can be helpful in diagnosing brain tumors prior to surgery, identifying small lesions within the brain, and in diagnosing and planning surgery across specialties. But many patients with existing neurosurgical implants (such as aneurysm clips, shunts for treating fluid build-up in the brain, and hardware for spinal surgery) are denied the benefits of 7T scanning out of compatibility and safety concerns.
A research team made up of Carle Illinois medical students, faculty, researchers from the Beckman Institute for
 Advanced Science and Technology, and clinicians at Carle Health is the first to delve into which of these devices has been tested with 7T MRI scanning. “We found that many implants have been tested at lower MRI fields (1.5T, 3T), but few have been adequately tested at 7T MRI,” said Annabelle Shaffer, a second-year medical student at Carle Illinois and study co-author. The testing results are particularly limiting in the clinical setting because patients with known neurosurgical issues are among the most likely to need MR imaging.
Advanced Science and Technology, and clinicians at Carle Health is the first to delve into which of these devices has been tested with 7T MRI scanning. “We found that many implants have been tested at lower MRI fields (1.5T, 3T), but few have been adequately tested at 7T MRI,” said Annabelle Shaffer, a second-year medical student at Carle Illinois and study co-author. The testing results are particularly limiting in the clinical setting because patients with known neurosurgical issues are among the most likely to need MR imaging. While the lack of widespread compatibility testing is discouraging, the news is better for some patients with certain neurosurgical implants. “We found that cranial fixation devices, deep brain stimulation devices, spinal rods, and pedicle screws are likely 7T MRI-compatible based on outcomes reported,” Shaffer said.
In 2021, Carle Foundation Hospital in Urbana became one of only a few hospitals in the country and the first in Illinois, to acquire the 7T MRI for both clinical and research use through a partnership with the University of Illinois Urbana-Champaign.
Shaffer said the team’s research is timely and may encourage implant device manufacturers to ensure that their devices are compatible with 7T and to test their products, using industry standards. “We hope more patients with biomedical implants (regardless of location) will be able to be safely imaged with this exciting technology,” Shaffer said.
The research team’s article “Neurosurgical Implant Safety in 7 T MRI: A Scoping Review,” was recently published in the Journal of Magnetic Resonance Imaging. Team members included: CI MED student Anant Naik; Carle Illinois College of Medicine Dean Dr. Mark Cohen; Dr. Paul M Arnold, a neurosurgeon at Carle Health and professor at Carle Illinois College of Medicine; Professor Brad Sutton of Carle Illinois College of Medicine and the Beckman Institute; Dr. David Weisbaum of Carle Health’s Department of Neurosurgery; and Aaron Anderson, Tracey Wszalek, Andrew Webb, and Bruce Damon of the Carle Illinois Advanced Imaging Center and the Beckman Institute.
Categories: Redefining Healthcare, Community
Tags: Carle Illinois Advanced Imaging Center, Carle Illinois College of Medicine, Champaign-Urbana, research